
This article briefly discusses the financial dealings of pharma companies related to the application of AI & D/MLor ANNin drug discovery and development processes as well as for optimization of analytical method conditions and formula of the dosage form.
Introduction
Producing pharmaceutical products without untoward effects but with desired qualities is not only the very basic requirement set by regulatory authorities but also indirectly decides the success of the pharma industry. The pharmaceutical product development process is a combined and coordinated work from numerous divisions of the pharma industry which usually starts from API discovery, synthesis and utilization to ends with formulation development, market positioning and successful use at the consumer end.
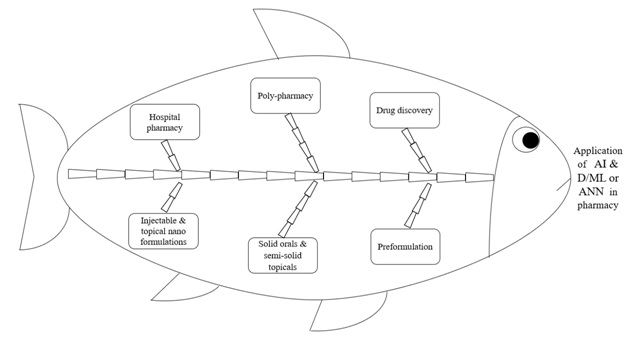
Conventional API discovery and development process, as well as optimization of analytical method and formula of dosage form, routinely use the quality by testing (QbT) approach or one factor at a time (OFAT) strategy. Since the time-consuming and chemical wastage in OFAT strategy or QbT approach is inevitable, looking for a better alternative approach or strategy which shows less time consumption in conjunction with minimal chemical wastage becomes an urgent requirement. In terms of the response surface methodology (RSM) approach, the design of experiment (DoE) is being utilized effectively to optimize APIs synthetic process, analytical method development and formula optimization for final pharmaceutical products. It should be added that the RSM-linked DoEis based on a linear model and therefore it won’t consider the non-linear modelization concept to see the influence of independent factors on the response variables. Thus, depending solely on the DoEfor such optimization processes may end with an erroneous conclusion and thus necessitates applying another approach (preferably of non-linear model-based) which judiciously eliminates the conclusion errors noticed with the DoE-based optimization process. One such non-linear model-based approach recently introduced in the pharmaceutical product development process is artificial intelligence and deep/machine learning (AI & D/ML) or artificial neural network (ANN). Non-linearity refers to a massive parallel network distributed throughout that allows for approximation and real-time operation to exhibit unpredictability and random behavior. The information processing capacity of ANN is related to the functioning of the normal human brain. Figure 1 shows the possible areas of pharmacy discipline wherein the AI & D/ML or ANN can be integrated.
Figure 1 Applications of AI & D/ML or ANN in the pharmacy field of the healthcare system
This article briefly discusses the financial dealings of pharma companies related to the application of AI & D/ML or ANNin drug discovery and development processes as well as for optimization of analytical method conditions and formula of the dosage form.
Pharma companies’ financial dealings
Table 1 displays the financial dealings of Pharma companies concerning the application part of AI & D/ML or ANN, particularly in drug discovery and development processes. The entry of AI & D/ML or ANN helps to shorten not only the new drug development period but also significantly minimizes the utilization of manpower and considerable reduction in expenditure related to the API development. For example, the German-based biotechnology company, Evotec, has partnered with a UK-based company, Exscientia, for the small molecule drug discovery process. Within a short period of 8 months, the discovered small drug molecule entered Phase 1 clinical trials which might usually have taken 4-5 years to deliver the drug candidate from the traditional drug discovery process (without utilizing AI & D/ML or ANN).
Table 1 Non-comprehensive financial dealings of pharma companies related to the application of artificial intelligence & deep/machine learning or artificial neural network in the drug discovery process (Partially taken from https://www.nature.com/articles/d43747-021-00045-7accessed on 21/07/ 2022)
| Companies | Headline |
| ZebiAI Therapeutics, Relay Therapeutics (Cambridge, USA) | Relay Therapeutics buys ZebiAI Therapeutics for $85 million and a further $185 million in potential revolutionary payments. |
| Recursion Pharmaceuticals (Salt Lake City, UT, US) | Recursion Pharmaceuticals finalizes $436 million initial public offering (IPO). |
| Exscientia (Oxford GB) | Exscientia gets an additional $225 million in a Series D round driven by SoftBank Vision Fund 2. |
| Exscientia (Oxford GB) | Exscientia finalizes $100 million Series C financing, with investors including Evotec, Bristol Myers Squibb and GT Healthcare. |
| Insitro (South San Francisco, CA, US) | Canada Pension Plan Investment Board supported in the financing of Insitro to raise $400 million in Series C. |
| Valo Health (Boston, MA, USA) | Valo Health, which is emerging its Opal computational drug discovery and development platform, increases $110 million to add to its $190 million upturned in January 2021 for its Series B funding round. |
| Roivant (Basel, New York), Silicon Therapeutics (Boston, USA) | Silicon Therapeutics was sold to Roivant for $450 million, including its physics-based platform for insilico small-molecule drug design, to be combined with Roivant’s machine learning approaches. |
| Cellarity (Cambridge, MA) | For the drug discovery approach related to modulating cellular behaviors, Cellarity raised $123 million in Series B funding. |
| AbCellera (Vancouver, Canada) | AbCellera ends its IPO at $556 million. |
| Recursion Pharmaceuticals (Salt Lake City, UT, US) | Recursion Pharmaceuticals, apply machine learning to cellular imaging data and raises $239 million in a Series D financing round directed by Bayer’s investment department Leaps. Other investors include Baillie Gifford, Casdin Capital, Samsara BioCapital and Lux Capital. |
| XtalPi (Cambridge, Massachusetts, US) | XtalPi is using quantum physics with AI to discover drug candidates and more than a dozen investment companies raised $318 million in a Series C round for this start-up. |
| Atomwise (San Francisco, CA, US) | Sanabil Invests around $123 million in Series B funding for Atomwise to upkeep the advancement of its molecule identification software. |
| Relay Therapeutics (Cambridge, USA) | Relay Therapeutics focused on protein motion to design drug candidates, calls a $400 million initial public offering IPO. |
| Insitro (South San Francisco, CA, US) | Insitro for its machine learning-based drug discovery approach raised $143 million in Series B funding. |
| AbCellera (Vancouver, Canada) | AbCellera for the expansion of its antibody drug discovery platform raised $105 million in Series B funding. |
| Schrödinger (New York City, USA) | Bill Gates and David Shaw’s Drug discovery software company ends a $232 million initial public offering (IPO). |
| Insitro (South San Francisco, CA, US), Gilead (Foster City, California, USA) | Gilead is the first big pharma company to sign a 3-year deal with Insitro for applying its Insitro Human platform to identify new drug targets for non-alcoholic steatohepatitis by producing experimental models of the disease. Insitro will be paid $15 million in a deal potentially worth $1 billion. |
AI & D/ML or ANN in optimization
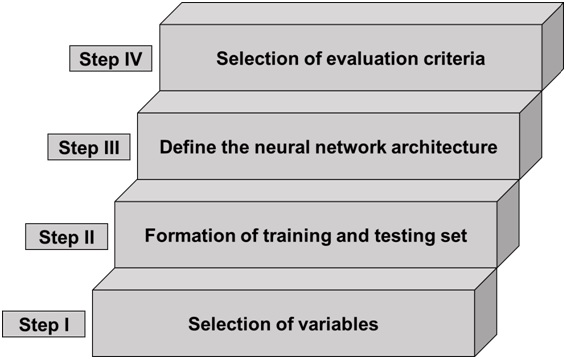
Before entering the discussion related to optimization of analytical method conditions and formula of dosage form,it needs to be emphasized that the ANN simply mimics the principles of information processing handled by the human brain wherein the influence of critical material attributes variation on critical analytical attributes (CAAs) can be predicted by segregating different sets of data (generated from numerous trails) into training, testing and validating. For this purpose, the ANN must be coupled with an algorithm to attain the “best fit” optimum values for a method.The ANN-linked algorithm produces a highly reliable and better predictor of the optimum values for a method than the RSM-based linear model. However, the ANN relies on the number of experiments/trials conducted and consequently, too high/a smaller number of trials would likely result in error and fault in the predictions.Therefore, the ANN takes the trials of the RSM-based linear modelfor generating the non-linear model [ANN-linked Levenberg-Marquardt (LM) algorithm] to predict the optimum regions for the studied CAAs.This type of integration between AI & D/ML or ANN and DoE allows the coining of new terminology called, “double-stage systematic optimization”. The double-stage systematic optimization was therefore started initially by using the conventional DoEapproach and then by the application of AI & D/ML or ANN. Furthermore, the ANN-linked LM is a potent chemometrics method because of its high performance and good prediction for non-linear systems.The typical network architecture of AI & D/ML or ANN is organized in three-different layers, viz., one input, one output and one or more hidden layers. Figure 2 portrays the schematic architecture of AI & D/ML or ANN having three input, ten hidden and three output layers (3:10:3). The architectural structure of AI & D/ML or ANN is the most common multi-layered perceptron (MLP) type which is built on four different elements, input, hidden and output layers along with connections or weights. Interestingly, the MLP type AI & D/ML or ANN works in two phases, training and testing. The training phase is based on the iterative demonstration of the available data pattern to teach the AI & D/ML or ANN for accomplishing the designated assignment. Figure 3 shows the various steps involved in developing the neural network. Other frequently used neuronal network combinations are the kohonen network, convolutional neural network (CNN) and recurrent neural network (RNN).
Figure 2 Schematic architecture of ANN having three input, ten hidden and three output layers
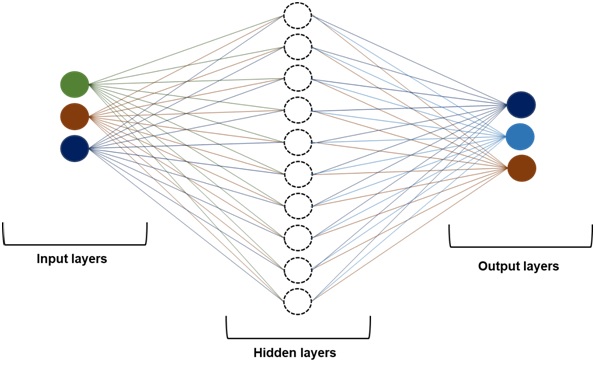
Figure 3 Schematic representation of the development of an AI & D/ML or ANN
Conclusion
The DoE (linear model)-supported AI & D/ML or ANN concept is currently being established to optimize not only the analytical method conditions for single or dual drug quantification but also the formula development for the dosage form. On the other hand, additional ways are also identified in recent years to omit the linear model support for AI & D/ML or ANN for intriguing pharmaceutical product development processes. To go ahead directly with non-linear models, the employment of multiple-input-multiple-output and multiple-input-single output-based ANN architecture, self-organizing map, MLP network trained with backpropagation algorithm, three- or multi-layer feed-forward ANN, etc. are being proposed currently by Yun et al. (2020) and Ramakrishna et al. (2014). Such kind of direct involvement of AI & D/ML or ANN will speed up the production performance as well as reduce human errors leading to improve the quality of pharmaceutical products.
References
RamaKrishna, K., Ramam, V. A., & Rao, R. S. (2014). Mathematical Neural Network (MaNN) Models Part VI: Single-layer perceptron [SLP] and Multi-layer perceptron [MLP] Neural networks in ChEM-Lab. Journal of Applicable Chemistry, 3(6), 2209-2311.
Yun, S., Kang, J. M., Kim, I. M., & Ha, J. (2020). Deep artificial noise: Deep learning-based precoding optimization for artificial noise scheme. IEEE Transactions on Vehicular Technology, 69(3), 3465-3469.